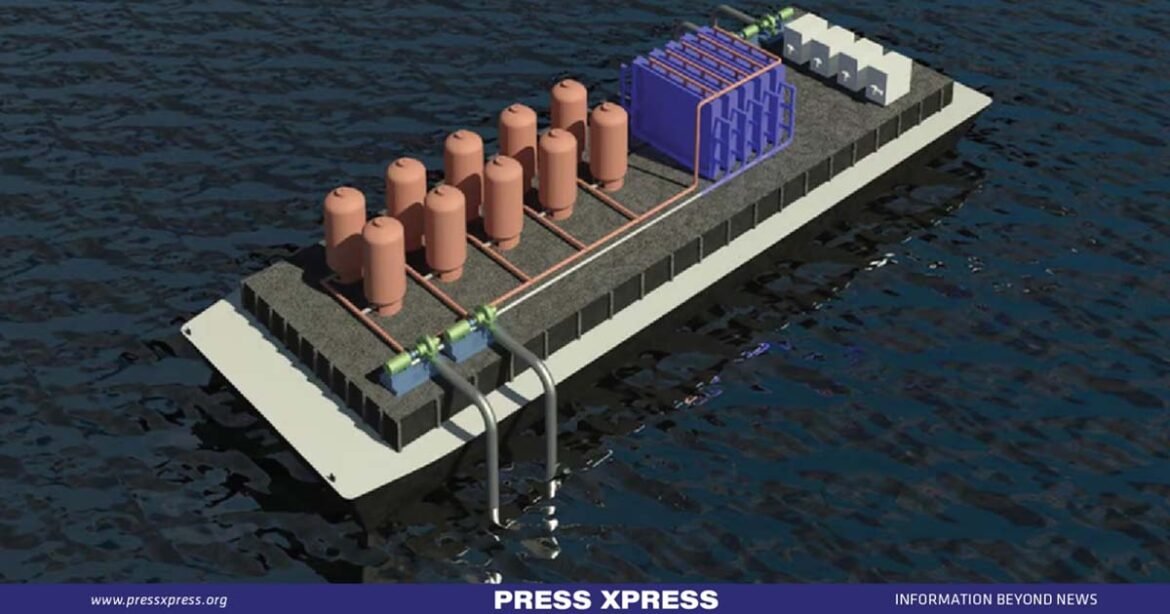
In a world where human activity and climate change have conspired to elevate carbon dioxide levels in our oceans, wreaking havoc on coral reefs and marine ecosystems, a group of dedicated researchers has unveiled a ground-breaking solution. They’ve harnessed the power of aqueous sodium hydroxide and sodium carbonate to combat this crisis, orchestrating a technological marvel that promises to reverse ocean acidification and mitigate global warming.
“It took years of relentless experimentation,” remarked Katherine Hornbostel, an esteemed assistant professor of mechanical engineering and materials science at the University of Pittsburgh’s Swanson School of Engineering, her voice tinged with the unmistakable fervor of achievement. “Observing the experimental outcomes align seamlessly with our meticulously developed models marked an unmatched moment of victory.”
YOU CAN ALSO READ: REVAMPING THE WAY WE GROW OUR FOOD: A CALL FOR TECHNOLOGICAL REDESIGN
A dual approach to ocean carbon capture
Hornbostel embarked on this extraordinary journey five years ago, when the concept of capturing carbon dioxide from the ocean was still in its infancy, lacking both research and funding. Her visionary approach has yielded two distinct models: one employing microencapsulated solvents comprised of sodium carbonate, and the other utilizing hollow fiber membrane contactors enriched with sodium hydroxide. These models, differing in geometry but united in purpose, offer a ray of hope for our imperiled oceans.

The first model, a brainchild of Hornbostel and Assistant Professor Tagbo Niepa from Swanson’s Department of Chemical and Petroleum Engineering, features capsules laden with a sodium solution-based solvent. Initially designed for medical applications, these capsules now serve as instrumental vessels for decarbonizing our precious ocean. Hornbostel likened these capsules to minuscule caviar beads, each encapsulating sodium carbonate solution, primed for the catalytic dance with carbon dioxide.
“In order to optimize the process,” Hornbostel explained with intensity, “one must densely pack these minute beads into the capillary tubes, providing an abundance of contact points. This allows more CO2 to seamlessly migrate from one capsule to another, drawn by the irresistible urge to react with the liquid held within.”
The ingenious sodium capsules have yet another ace up their sleeve – regeneration. Subjected to steaming at temperatures ranging from 100 to 120 degrees Celsius, these capsules release their captive carbon dioxide, ready for storage. This regeneration process not only ensures efficient carbon dioxide removal but also extends the capsules’ lifespan, enabling them to embark on multiple cycles of carbon capture.
The second model, employing the “hollow fiber membrane,” functions as a conduit for seawater, ushering it into contact with the sodium hydroxide solvents housed within cylindrical capsules. This innovative membrane facilitates the seamless exchange between seawater and the solvent, offering a conduit for mass transfer. Crafted from a flexible polymer akin to polypropylene, the hollow fiber membrane permits the passage of gases while resolutely repelling liquids and ions. The research team proudly claims to be the pioneers in showcasing CO2 removal from seawater through the membrane contactor method, marking a pivotal milestone in their journey towards a healthier planet.
Hornbostel emphasized the paramount importance of a high surface area for seawater, elucidating its critical role in expeditious carbon dioxide removal from the water. “The rate of mass transfer is directly proportional to surface area,” she expounded, her tone exuding authority. “Increasing the surface area of a membrane by a factor of two directly corresponds to a twofold enhancement in the rate at which carbon dioxide penetrates it.”
The race to prevent a 1.5-degree Celsius rise
The promise of direct ocean capture technology lies in its potential to access the substantial reservoir of bound CO2 within seawater. What’s more, the inherent density of seawater, a thousandfold greater than that of air, implies the feasibility of constructing more compact systems. The concept even entertains the possibility of offshore deployment, on disused oil platforms, for instance, saving valuable terrestrial space and synergizing with offshore wind energy and CO2 storage initiatives.
Hornbostel candidly acknowledged the formidable challenge of upscaling the technology to operate affordably on a larger oceanic scale. To address this, she and her team are exploring techniques to elevate the pH of ocean water, a catalyst for enhanced carbon dioxide release. Although the specifics remain in the planning stage, envisaging the placement of these models in regions with high oceanic carbon dioxide concentrations is within the realm of possibility.
As David Koweek, chief scientist at OceanVisions, aptly pointed out, “The ocean, as the largest component of Earth’s natural carbon cycle, holds immense potential for gigaton-scale carbon dioxide removal.” Yet, this potential has remained largely untapped due to insufficient research and development in ocean-based carbon dioxide removal technologies.

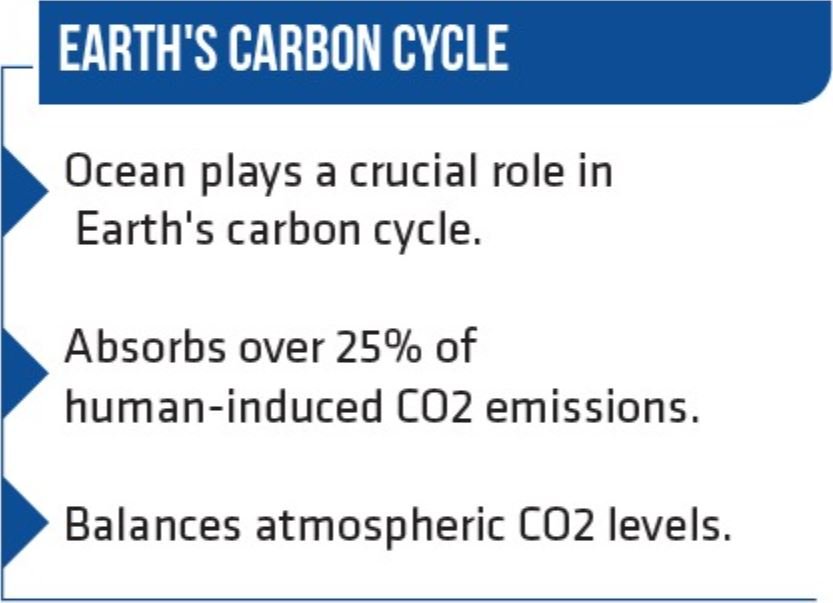
Hornbostel’s research underscores the imperative of removing 10 gigatons of carbon dioxide from the environment annually by 2050 to safeguard against a temperature rise beyond the ambitious 1.5 degrees Celsius target set by the 2050 Paris Climate Agreement. Notably, studies have illuminated that the ocean blankets over 70 percent of Earth’s surface, absorbing approximately a quarter of human-induced carbon dioxide emissions. This intricate dance between the atmosphere and ocean continually exchanges carbon dioxide and various other gases. Hornbostel astutely emphasized that carbon capture technology transcends both ocean and atmospheric domains, characterizing it as “indirect air capture.” In this symbiotic process, the ocean, lying beneath, draws more carbon dioxide from the air above to replenish the carbon dioxide removed.
However, the consequences of excessive carbon dioxide influx into the ocean are dire. As carbon dioxide from the atmosphere dissolves into the ocean, it transforms into carbonic acid, subsequently lowering the ocean’s pH levels. This acidification renders the marine environment less hospitable for aquatic life, a sobering fact affirmed by the U.S. Department of Commerce’s National Oceanic and Atmospheric Administration.
Peter Petraitis, a distinguished professor of biology at the University of Pennsylvania, has meticulously tracked a distressing trend in the Gulf of Maine over the past two decades: a disconcerting correlation between elevated dissolved carbon dioxide levels in the ocean and a precipitous decline in mussels and gastropods. Even more disquieting is the recent revelation from Petraitis and his research team that gastropod populations are dwindling at an alarming rate, surpassing their initial projections.
As Petraitis succinctly puts it, “The evidence is compelling; as water temperatures rise, gastropod populations decline. The correlation is undeniably robust.”
Ocean acidification: A looming crisis
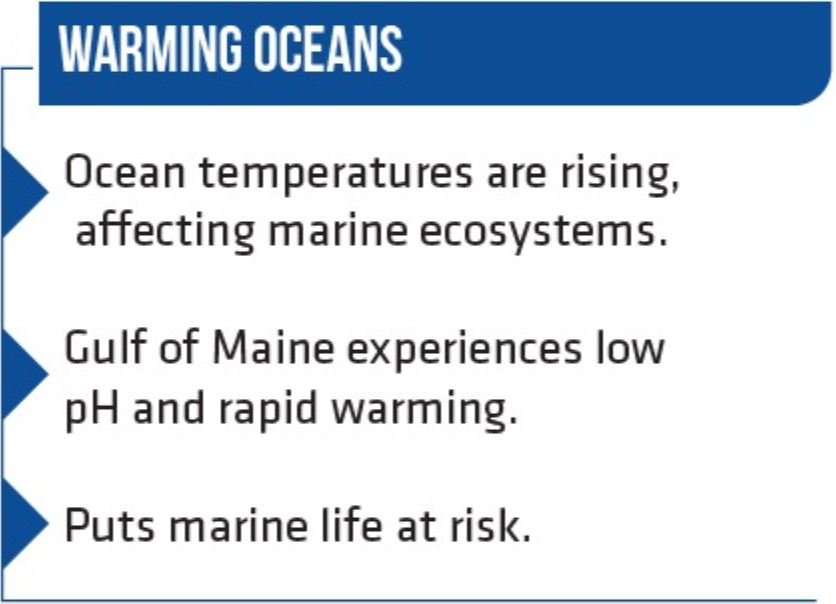
Petraitis’s comprehensive research paints a bleak picture for the Gulf of Maine. This region is grappling with low pH levels and a rate of warming that exceeds a staggering 99.9 percent of all global oceans. As time marches forward, the Gulf of Maine’s capacity to mitigate the pernicious effects of ocean acidification is rapidly diminishing.

The current global ocean pH level hovers at approximately 8.1, reflecting a troubling drop of 0.1 since the onset of the Industrial Revolution, as reported by the Environmental Protection Agency. This seemingly modest shift in pH is deeply concerning, as it translates to a tenfold increase in acidity for each unit decrease. This subtle acidification, a byproduct of carbon dioxide dissolution in water, disrupts the delicate balance of calcium carbonate and bicarbonate in the ocean. These compounds are essential building blocks for the creation of the rigid shells and skeletons vital to the survival of corals and various invertebrates.
In light of these grave findings, Hornbostel’s research team’s technology offers a glimmer of hope. However, it comes with a crucial caveat: it necessitates the large-scale production of capsules and demands a comprehensive exploration of its compatibility with existing infrastructure, such as desalination facilities. Their research paper passionately advocates for a deeper commitment to investigating seawater carbon dioxide capture technology. This call to action is nothing short of imperative, given the relentless pace of ocean warming and acidification, which threaten to disrupt the delicate equilibrium of our marine ecosystems.