France has positioned itself as a significant player in the global effort to address the challenges posed by rare diseases. These conditions, which affect fewer than one in 2,000 individuals, have historically been underserved in terms of medical research and treatment options. However, French pharmaceutical companies and research institutions have increasingly focused on finding solutions, leveraging innovation and collaboration to develop new treatments.
The Landscape of Rare Diseases in France
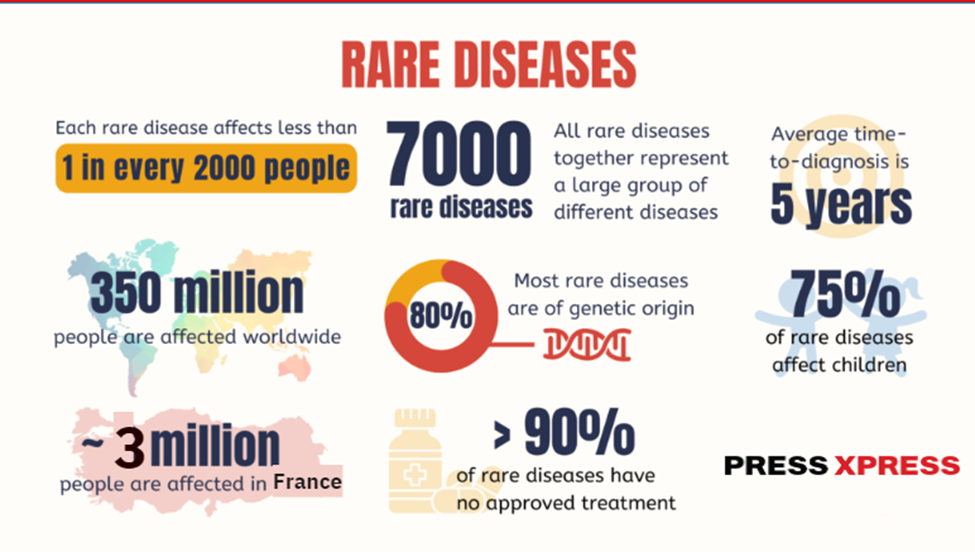
Rare diseases impact approximately 3 million people in France, contributing to a global population of over 300 million affected by these conditions, according to EURORDIS (European Organisation for Rare Diseases). With more than 7,000 distinct rare diseases identified globally, 95% remain without approved treatments, highlighting the significant gap in therapeutic solutions.
France initiated its first National Plan for Rare Diseases in 2004, with successive plans aiming to improve diagnosis, treatment, and research. The third iteration of this plan (2018-2022) set ambitious goals, including reducing diagnostic delays and expanding research infrastructure. As of 2023, official figures from the French Ministry of Health show that the country has established 23 national reference centers and over 400 centers of expertise, dedicated to advancing rare disease research and improving patient care.
French Pharmaceutical Innovations in Rare Disease Treatment
Several French pharmaceutical companies have been at the forefront of global efforts to develop treatments for rare diseases. Sanofi, one of France’s largest pharmaceutical companies, has made considerable progress in this field. In 2023, Sanofi’s therapy Avalglucosidase alfa, used to treat Pompe disease, received approval from the European Medicines Agency (EMA), a significant milestone in managing this rare metabolic disorder, which affects fewer than 1,000 people in France. Sanofi is also advancing clinical trials for Venglustat, a potential treatment for Fabry disease, another rare genetic condition.
Ipsen, a prominent French biopharmaceutical company, has focused on treating rare neuroendocrine tumors. Its flagship drug, Somatuline (lanreotide), which targets these rare cancers, has contributed to improved survival rates for patients. Ipsen’s work extends into other rare conditions, particularly those affecting the nervous system, aligning with its strategy to focus on niche markets.
Another significant player in France is Genethon, a biotech firm specializing in gene therapy for rare neuromuscular disorders. In collaboration with international research bodies, Genethon has advanced clinical trials in gene therapies targeting Duchenne muscular dystrophy.
Collaborative Efforts: Bridging Academia and Industry
France’s strategy for tackling rare diseases has been characterized by strong collaborations between academia, industry, and government bodies. The creation of France’s Medicines Innovation Hub has fostered partnerships between pharmaceutical firms, biotech startups, and research institutes.
On a broader scale, France has played a leading role in the European Joint Programme on Rare Diseases (EJP RD), which aims to enhance cooperation between research institutions across Europe.
France’s participation in Horizon Europe, the European Union’s research and innovation funding program, has further strengthened its research capacity. By securing access to continental funding and collaborative networks, France has bolstered its efforts to find new treatments for rare diseases through multinational projects.
Data and AI’s Role in Rare Disease Innovation
In recent years, artificial intelligence (AI) and big data have become increasingly important in France’s rare disease research. According to the Institut National de la Santé et de la Recherche Médicale (INSERM), AI technologies are significantly shortening the diagnostic process for rare diseases, which can typically take several years due to the complexity and rarity of symptoms. By analyzing vast amounts of genetic and clinical data, AI platforms can detect patterns that may lead to faster diagnoses and more targeted treatments.
Pharmaceutical companies, including Sanofi, have been using AI to streamline drug discovery processes and optimize clinical trials. These AI-driven initiatives help reduce the time required to bring new therapies to market and enable more personalized approaches to rare disease treatment, where one-size-fits-all solutions are often ineffective.
Economic and Regulatory Challenges
The development of treatments for rare diseases faces several economic and regulatory hurdles. Rare disease drug development is costly, with high research and development expenses coupled with small patient populations, limiting the potential return on investment for pharmaceutical companies. However, regulatory frameworks such as the EU’s orphan drug designation provide incentives, including market exclusivity and tax benefits, to encourage investment in rare disease research.
In 2023, the European Medicines Agency (EMA) granted several orphan drug designations to French pharmaceutical firms, including Sanofi and Ipsen, for their work on novel treatments for rare diseases.
The cost of rare disease drugs remains a challenge for healthcare systems. According to a 2023 report from the French National Health Insurance (CNAM), the high cost of some treatments, which can exceed €500,000 per patient annually, has prompted debates over funding models and equitable access to these life-saving therapies.
The Future of Rare Disease Treatment in France
France’s approach to rare disease treatment, grounded in innovation, collaboration, and data-driven solutions, has positioned it as a leader in this field. However, significant challenges remain, particularly in ensuring that new therapies are accessible and affordable for all patients. The continued integration of AI and advancements in gene therapy offer hope for more effective and personalized treatments, but the high costs associated with these innovations may limit their accessibility.
Future efforts will need to focus on addressing these economic challenges while maintaining the momentum of scientific progress. Ensuring equitable access to rare disease treatments, while balancing the financial sustainability of healthcare systems, will be a key issue moving forward.


