Two scientists have won the Nobel Prize in medicine for discoveries that enabled the creation of mRNA vaccines against COVID-19 and that could be used to develop other shots in the future
Two scientists, Hungarian-American Katalin Kariko and her American colleague Drew Weissman, have been honored with the 2023 Nobel Prize in medicine for their collaborative efforts in pioneering the revolutionary technology behind some of the most potent COVID-19 vaccines. This prestigious recognition highlights their groundbreaking work, which commenced in the early 1990s at the University of Pennsylvania, focusing on “mRNA” technology. Their pioneering contributions were instrumental in the development of the highly effective COVID-19 vaccines by Moderna and Pfizer-BioNTech.
Lifesaving vaccines
The Nobel Prize in Medicine Committee in Sweden stated that this discovery has played a pivotal role in overcoming one of the most significant contemporary threats to human health.
Rickard Sandberg, a member of the Nobel committee, addressed reporters on Monday following the announcement, saying, “mRNA vaccines, in conjunction with other COVID-19 vaccines, have been administered more than 13 billion times. Collectively, they have saved countless lives, prevented severe cases of COVID-19, reduced the overall disease burden, and facilitated the reopening of societies.”
Sandberg continued, “mRNA technologies are now being harnessed for the development of vaccines against various other infections. Additionally, there is hope that this technology can be employed for therapeutic protein delivery and cancer treatment, with the potential to further enhance human health.”
Director-General Tedros Adhanom Ghebreyesus of the World Health Organization offered his congratulations to the Nobel Prize recipients on Monday, remarking, “Today marks a momentous day for healthcare, science, and the field of vaccines.” He made these comments during a press briefing in Geneva.
mRNA vaccines: A promising idea
Inside our cells, genetic information stored in DNA is translated into messenger RNA (mRNA), serving as a blueprint for protein synthesis. In the 1980s, the advent of efficient methods for generating mRNA outside of cellular environments, known as in vitro transcription, marked a pivotal breakthrough, propelling molecular biology advancements across various domains.
During this period, the concept of harnessing mRNA technology for vaccines and therapies emerged, but it faced significant hurdles. In vitro transcribed mRNA was perceived as unstable and challenging to deliver, necessitating the development of intricate lipid-based carrier systems for mRNA encapsulation. Additionally, in vitro-produced mRNA triggered inflammatory reactions, dampening enthusiasm for its clinical potential.
Despite these obstacles, Hungarian biochemist Katalin Karikó remained undeterred in her mission to unlock the therapeutic potential of mRNA. In the early 1990s, while serving as an assistant professor at the University of Pennsylvania, she persevered in her vision, even when faced with challenges in persuading research funders of her project’s significance. Drew Weissman, an immunologist and her new colleague at the university, shared a common interest in dendritic cells, crucial players in immune surveillance and vaccine-induced immune responses. This synergy sparked a fruitful collaboration between the two, centered on investigating how different types of RNA interacted with the immune system.
Karikó and Weissman’s Pioneering Research
In their research, Karikó and Weissman observed that dendritic cells treated in vitro transcribed mRNA as foreign, causing an inflammatory response. They hypothesized that chemical modifications in the bases of mammalian cell mRNA might be the reason for this difference. To test this, they created various mRNA variants with unique base modifications and found that adding these modifications reduced the inflammatory response significantly. This discovery, made in 2005, revolutionized our understanding of mRNA’s recognition by cells and had profound implications for mRNA therapy, predating the COVID-19 pandemic by 15 years.
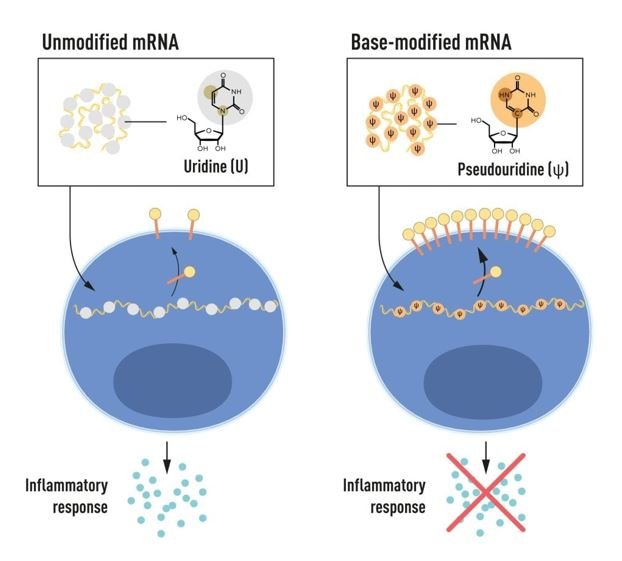
In subsequent studies published in 2008 and 2010, Karikó and Weissman demonstrated that modified mRNA also greatly increased protein production compared to unmodified mRNA by reducing the activation of an enzyme that regulates protein synthesis. Their findings not only reduced inflammatory responses but also enhanced protein production, paving the way for the clinical use of mRNA technology.
Future applications
Weissman expressed his enthusiasm for the incredible future potential of mRNA technology, stating, “We’ve contemplated the possibilities of RNA for years, and now it’s a reality,” as he conveyed to the Associated Press.
The emergence of the coronavirus pandemic expedited the advancement of mRNA technology, as noted by Paul Hunter, a professor of medicine at the University of East Anglia in the United Kingdom. Hunter commented, “Before COVID, there was awareness of ongoing work on mRNA vaccines, but achieving real-world application of this technology seemed distant. Now that it has proven to be effective, potentially surpassing many other vaccine types, it has received a significant boost. The technology holds promise for a wide range of applications.”
Hunter also highlighted the rapid pace of development in this field, stating, “The process of creating new vaccines is now considerably easier and faster.”
Katalin Karikó, born in 1955 in Szolnok, Hungary, earned her Ph.D. from Szeged’s University in 1982 and conducted postdoctoral research at the Hungarian Academy of Sciences in Szeged until 1985. Subsequently, she pursued postdoctoral research at Temple University in Philadelphia and the University of Health Science in Bethesda. In 1989, she assumed the role of Assistant Professor at the University of Pennsylvania, where she remained until 2013. Following that, she held positions as Vice President and later Senior Vice President at BioNTech RNA Pharmaceuticals. Since 2021, she has served as a Professor at Szeged University and an Adjunct Professor at the Perelman School of Medicine at the University of Pennsylvania.
Drew Weissman, born in 1959 in Lexington, Massachusetts, USA, attained his MD and PhD degrees from Boston University in 1987. He completed his clinical training at Beth Israel Deaconess Medical Center at Harvard Medical School and engaged in postdoctoral research at the National Institutes of Health. In 1997, Weissman established his research group at the Perelman School of Medicine at the University of Pennsylvania. He currently holds the position of Roberts Family Professor in Vaccine Research and serves as the Director of the Penn Institute for RNA Innovations.


